16. Zahn LE, Gannon PM, Rajakovich LJ*. Iron-sulfur cluster-dependent enzymes and molybdenum-dependent reductases in the anaerobic metabolism of human gut microbes. Metallomics 2024, 16(11): 1-16.
https://academic.oup.com/metallomics/article/16/11/mfae049/7879537
15. Perkins SW, Hlaing MZ, Hicks KA, Rajakovich LJ*, Snider MJ*. Mechanism of the multi-step catalytic cycle of 6-hydroxynicotinate 3-monooxygenase revealed by global kinetic analysis. Biochemistry 2023, 62(10): 1553-1567.
https://doi.org/10.1021/acs.biochem.2c00514
Prior to UW (up to 2022)
14. Rajakovich LJ, Fu B, Bollenbach M, Balskus EP. Elucidation of an anaerobic pathway for metabolism of l-carnitine-derived gamma-butyrobetaine to trimethylamine by human gut bacteria. Proc. Natl. Acad. Sci. 2021, 118(32): e2101498118.
https://doi.org/10.1073/pnas.2101498118
bioRxiv preprint: https://doi.org/10.1101/2021.01.25.428109
13. Rajakovich LJ, Zhang B, McBride MJ, Boal AK, Krebs C, Bollinger JM, Jr. Emerging structural and functional diversity in proteins with dioxygen-reactive dinuclear transition metal cofactors. In Comprehensive Natural Products III – 3rd Edition: Chemistry & Biology, Liu H-w & Begley T, Eds. Elsevier, 2020, 215-250. (book chapter)
doi: 10.1016/B978-0-12-409547-2.14864-4
12. Zhang B#, Rajakovich LJ#, Van Cura D, Blaesi EJ, Mitchell AJ, Tysoe CR, Zhu X, Streit BR, Rui Z, Zhang W, Boal AK, Krebs C, Bollinger JM Jr. Substrate-triggered formation of a peroxo-Fe2(III/III) intermediate in the fatty acid decarboxylation by UndA. J. Am. Chem. Soc. 2019, 141(37): 14510-14514.
#equal contribution
https://doi.org/10.1021/jacs.9b06093
11. Rajakovich LJ, Pandelia M-E, Mitchell AJ, Chang W-c, Zhang B, Boal AK, Krebs C, Bollinger JM Jr. A new microbial pathway for organophosphonate degradation by two previously misannotated non-heme-iron oxygenases. Biochemistry 2019, 58(12): 1627-1647.
https://doi.org/10.1021/acs.biochem.9b00044
10. Rajakovich LJ, Balskus EP. Metabolic functions of the human gut microbiota: the role of metalloenzymes. Nat. Prod. Rep. 2019, 36(4): 593-625. (review)
https://doi.org/10.1039/c8np00074c
9. Chekan JR, Ongpipattanakul C, Wright TR, Zhang B, Bollinger JM Jr, Rajakovich LJ, Krebs C, Cicchillo R, Nair SK. Molecular basis for enantioselective herbicide degradation imparted by aryloxyalkanoate dioxygenases (AADs) in transgenic plants. Proc. Natl. Acad. Sci. USA 2019, 116(27): 13299-13304.
https://doi.org/10.1073/pnas.1900711116
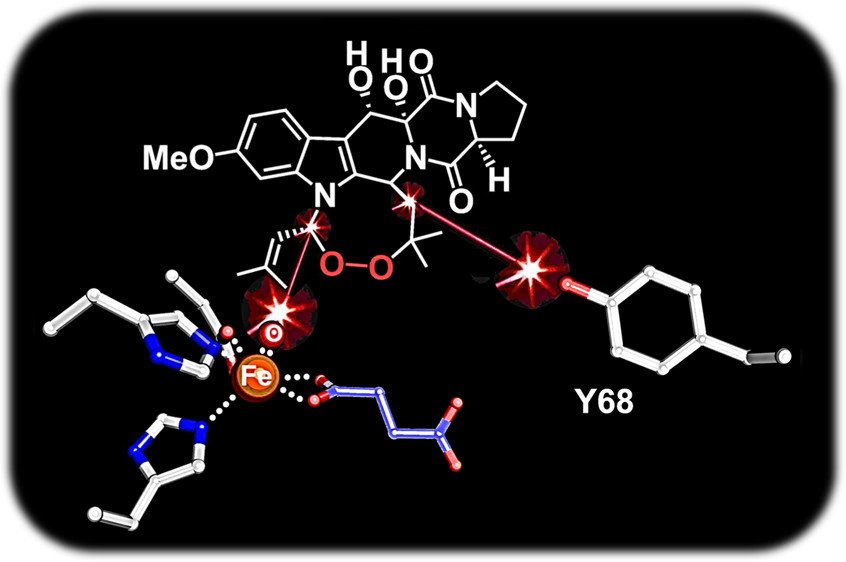
8. Dunham NP, Del Río Pantoja J, Zhang B, Rajakovich LJ, Allen BD, Krebs C, Boal AK, Bollinger JM Jr. Hydrogen donation but not abstraction by a tyrosine (Y68) during endoperoxide installation by verruculogen synthase (FtmOx1). J. Am. Chem. Soc. 2019, 141(25): 9964-9979.
https://doi.org/10.1021/jacs.9b03567
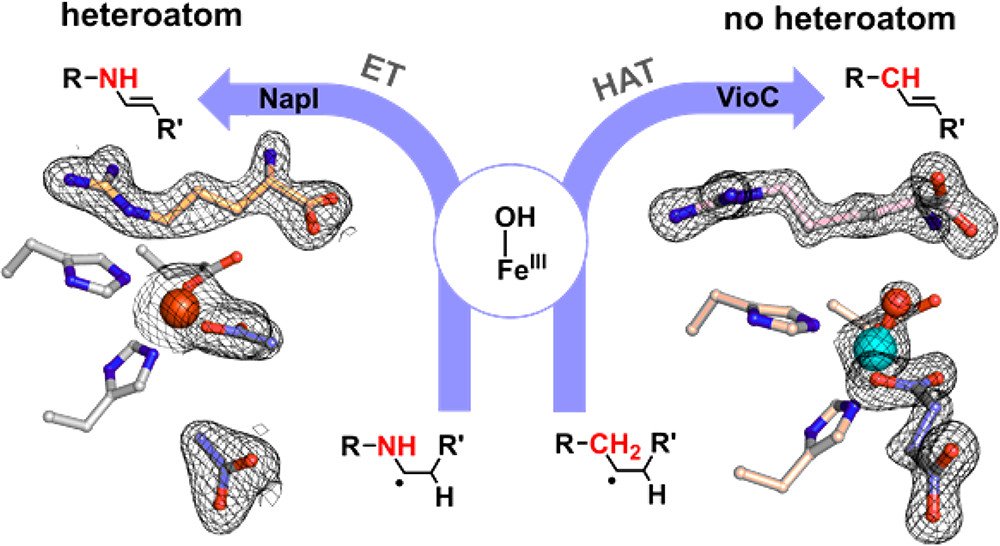
7. Dunham NP, Chang W-c, Mitchell AJ, Martinie RJ, Zhang B, Bergman JA, Rajakovich LJ, Wang B, Silakov A, Krebs C, Boal AK, Bollinger JM Jr. Two distinct mechanisms for C–C desaturation by iron(II)- and 2-(oxo)glutarate-dependent oxygenases: Importance of α-heteroatom assistance. J. Am. Chem. Soc. 2018, 140(23): 7116-7126.
https://doi.org/10.1021/jacs.8b01933
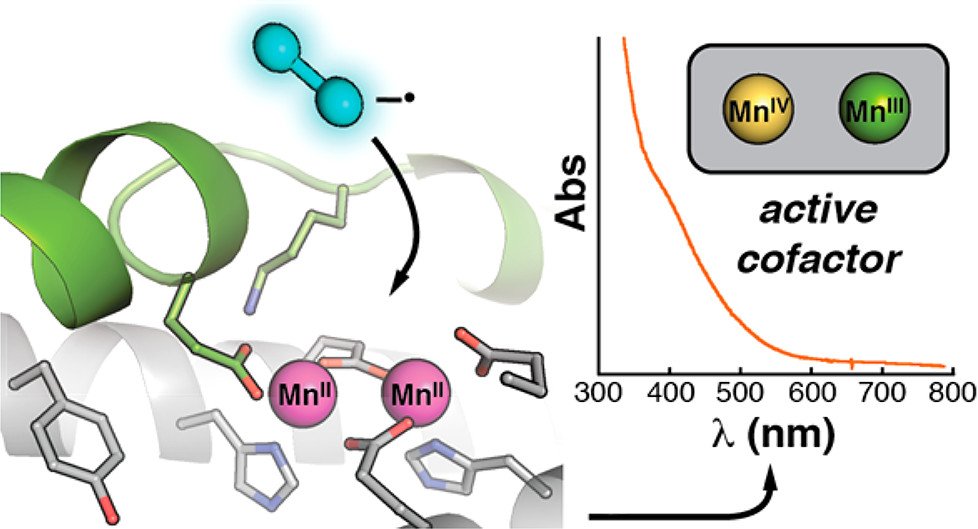
6. Rose HR, Ghosh MK, Maggiolo AO, Pollock CJ, Blaesi EJ, Hajj V, Wei Y, Rajakovich LJ, Chang W-c, Han Y, Hajj M, Krebs C, Silakov A, Pandelia M-E, Bollinger JM Jr., Boal AK. Structural basis for superoxide activation of Flavobacterium johnsoniae class I ribonucleotide reductase and for radical initiation by its dimanganese cofactor. Biochemistry 2018, 57(18): 2679-2693.
https://doi.org/10.1021/acs.biochem.8b00247
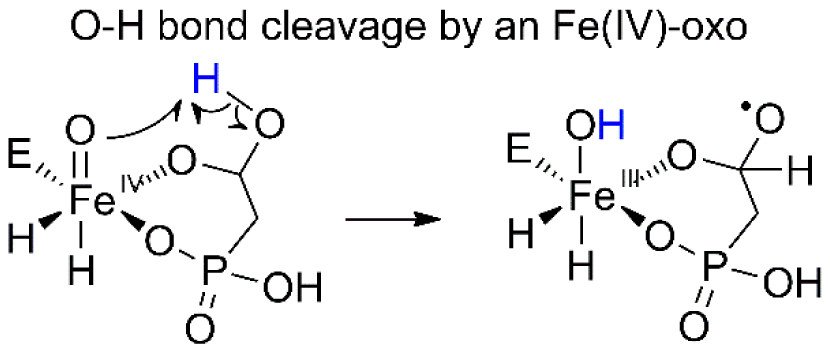
5. Peck SC, Wang C, Dassama LM, Zhang B, Guo Y, Rajakovich LJ, Bollinger JM Jr., Krebs C, van der Donk WA. O–H activation by an unexpected ferryl intermediate during catalysis by 2-hydroxyethylphosphonate dioxygenase. J. Am. Chem. Soc. 2017, 139(5): 2045-2052.
https://doi.org/10.1021/jacs.6b12147
4. Rajakovich LJ, Nørgaard H, Warui DM, Chang W-c, Li N, Booker SJ, Krebs C, Bollinger JM Jr., Pandelia M-E. Rapid reduction of the diferric-peroxyhemiacetal intermediate of aldehyde-deformylating oxygenase by a cyanobacterial ferredoxin: evidence for a free-radical mechanism. J. Am. Chem. Soc. 2015, 137(36): 11695-11709.
https://doi.org/10.1021/jacs.5b06345
3. Warui DM, Pandelia M-E, Rajakovich LJ, Krebs C, Bollinger JM Jr., Booker SJ. Efficient delivery of long-chain fatty aldehydes from the Nostoc punctiforme acyl-acyl carrier protein reductase to its cognate aldehyde-deformylating oxygenase. Biochemistry 2015, 54(4): 1006-1015.
https://doi.org/10.1021/bi500847u
2. Pandelia M-E, Li N, Nørgaard H, Warui DM, Rajakovich LJ, Chang W-c, Booker SJ, Krebs C, Bollinger JM Jr. Substrate-triggered addition of dioxygen to the diferrous cofactor of aldehyde-deformylating oxygenase to form a diferric-peroxide intermediate. J. Am. Chem. Soc. 2013, 135(42): 15801-15812.
https://doi.org/10.1021/ja405047b
1. Rajakovich LJ, Tomlinson J, Dos Santos PC. Functional analysis of Bacillus subtilis genes involved in the biosynthesis of 4-thiouridine in tRNA. J. Bacteriol. 2012, 194(18): 4933-4940.
https://doi.org/10.1128/jb.00842-12